The Pingping Tang Group @ NKU
State Key Laboratory of Elemento-organic ChemistryTel:
Add: Nankai University, Tianjin 300071, P. R. China
Copyright © 2022 Prof. Pingping Tang Research Group
Introduction
Research in our group explores the boundaries of fluorinating organic synthesis to enable the more rapid creation of fluorinated molecules in a predictable and controllable fashion. We are particularly inspired by natural products not only because of their importance as synthetic targets but also due to their ability to serve as invaluable promoters for benefits of the human society and the development of human civilization.
We are interested in the practical total synthesis of natural products for which there is true impetus for their construction due to potential pharmaceutical application. Another major focus of our research is selective trifluoromethoxylation of organic molecules, yet methods capable of accessing fluorinated motifs in a chemo-, regio-, and enantioselective fashion are lacking. More importantly, our ultimate goal is to combine the total synthesis of natural products to synthesis fluorinated derivatives for new drug development.
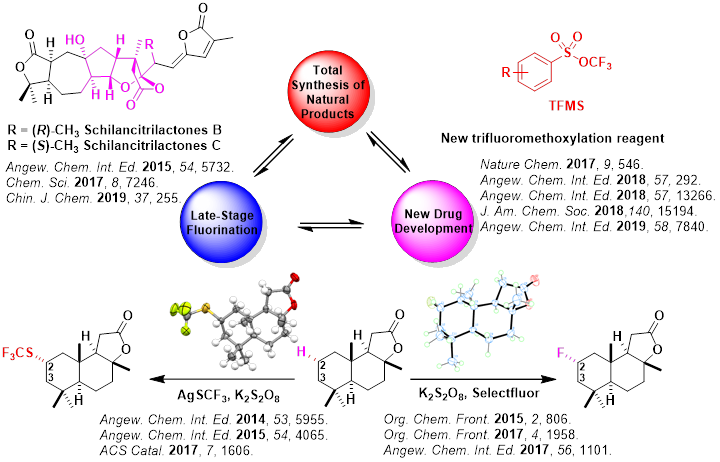
Team A: Total Synthesis of Natural Products
(Read our representative work on total synthesis)
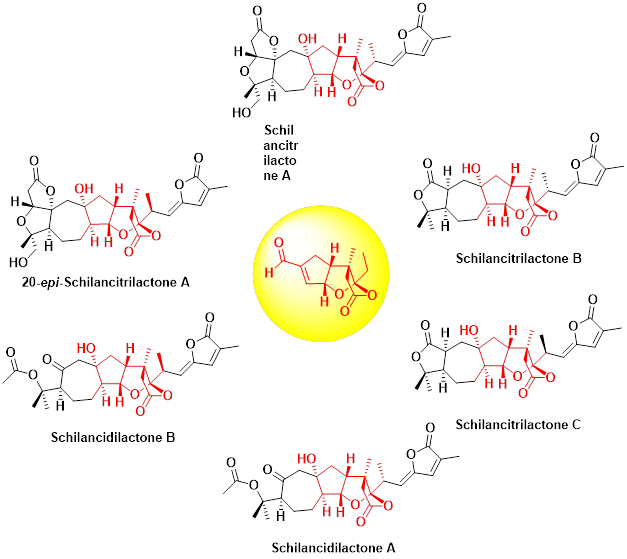
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially available precursors. We particularly focus on the medicinally important active ingredients, which could be further fluorinated for biological activity testing. The The total synthesis of Schilancidilactones A, B and Schilancitrilactones A, B, C has been accomplished from the common intermediates for the first time by our group. An intramolecular radical cyclization, late-stage halogenation and AIBN-mediated or Ni-catalyzed intermolecular radical cross coupling reaction were employed as the key steps.
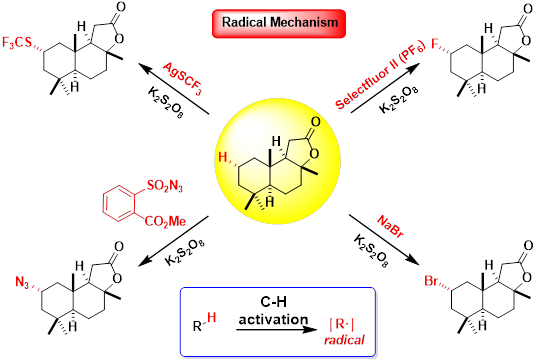
Team B: Modern Organic Fluorination
(Read our representative work on organic synthesis)
The historical methods for introduction the fluorine are often harsh, and are not suitable for the late-stage functionalization of complex molecules. Therefore, it’s of great necessity and urgency to develop new methods and brand new reagents of high efficiency and high selectivity for the synthesis of fluorinated complex molecules in the mild conditions.
1) Late-Stage Fluorination
The C–H functionalization field has transformed the way that chemists approach the synthesis of natural products and bioactive molecules, enabling fast and efficient derivatization. Our group is focused on the development of fluorination methods to enable the selective C–H functionalization of a variety of different substrates, ranging from simple, commodity chemicals to complex natural products and pharmaceuticals.
2) Development of Novel Trifluoromethoxylating Reagent
· The Trifluoromethyl Arylsulfonate(TFMS) has been greatly explored as trifluoromethoxylating reagent for a great deal of transformations in our group, which is bench stable, easily handled and favorably triggered by nucleophiles to deliver CF3O- in situ.
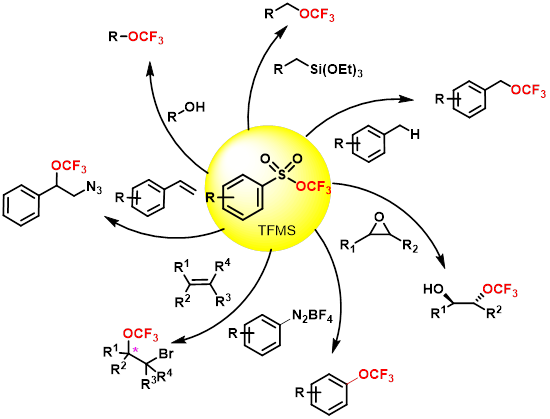
· More practical reagents to generate CF3O radical and cation are under development.
Team C: New Drug Development:
Due to the special properties of fluorine atoms, the introduction of fluorine atoms can dramatically change the electrical properties of molecules and increase the stability of molecular metabolism and fat solubility. Preliminary bioassay shows some of schinortriterpenoid possess fascinating bioactivities, such as antitumor, antihepatitis, anti-HIV-1. With the practical synthesis routes in hand, a wild range of complicated fluorinated compounds has been successfully synthesized in high yield and excellent enantioselectivity in our group. The biological activity testing is in progress.